AIDC in Healthcare |
ماهنامه شماره10(خرداد ماه 1395)
Introduction -1
“Automatic Identification and Data Capture” (AIDC) refers to the methods of automatically identifying objects, collecting data about them, and entering that data directly into computer systems without human involvement.
The implementation of AIDC systems is planned to improve patient safety, reduce medication errors, facilitate anti-counterfeiting and enable effective product recalls, and adverse event reporting, while addressing inefficiencies throughout the Healthcare supply chain, and allowing stakeholders to improve and integrate their processes. The goal of Healthcare AIDC application standards is to define the data to carry, using specific data carriers, for every healthcare product, at every packaging level.
The vision of GS1 Healthcare is to be the recognised, open and neutral source for regulatory agencies, industry organisations and other similar stakeholders who are seeking direction for global standards in healthcare for patient safety, supply chain security & efficiency, traceability and accurate data synchronisation. The vision of AIDC is that every item has one set of key identification data carried in one data carrier able to be scanned by everyone at every key process step. The mission of GS1 Healthcare is to lead the Healthcare sector to the successful development and implementation of global standards by bringing together experts in Healthcare to enhance patient safety and supply chain efficiencies.
The result of using AIDC (Automatic Identification and Data Capture) Standards for Healthcare trade items is providing Healthcare industry stakeholders with a common set of data and data carriers to be applied to medical products at every packaging level, including specific selection and use of:
- Appropriate GS1 Product Identification Keys
- Additional product and production data, for example; batch/lot, expiration date, and/or serial number (where applicable)
- GS1 Data Carriers including; linear barcodes, two-dimensional barcodes and Radio Frequency Identification (RFID) tags
Product types – 2
All items in healthcare can be classified in two main groups; Pharmaceutical Products and Medical Devices.
2-1. Pharmaceutical Products
These products are submitted for market authorisation to be sold in a country. Depending on the healthcare organisation, an authorisation code can be given by a government agency, a GS1member organisation, or any organisation validated by the ministry of health to allocate this code. In most countries these products can only be sold in a selected distribution channel such as pharmacies and are subject to specific regulations.
2-1-1. Over the Counter (OTC)
An OTC is a pharmaceutical product, drug, or medicinal specialty who’s dispensing or administration does not require medical authorisation. Normally it can be used by the consumers under their own initiative and responsibility to prevent, relieve or to treat symptoms or mild diseases. Its use, in the form, conditions and authorised dosages should be safe for the consumer.
This covers healthcare items, pharmaceuticals, and medical devices that do not require a medical prescription or direct medical intervention. Typical examples include bandages, first-aid kits, mouthwash, low-strength pain-killers, etc.
2-1-2. Medical Prescription (Rx)
A Medical Prescription Product (Rx) is a drug, medical device, or medicinal specialty that requires a medical prescription or direct medical intervention.
Typical examples include, medicated bandages, pain medication, injectables, etc. and can normally only be obtained with a prescription from an appropriate healthcare practitioner.
2-1-3. Hospital Pharmacy Production
A Hospital Pharmacy Product is a product that has to be manufactured by a hospital pharmacy for internal or multi-hospital use, thus it is not (or is no more) marketed by the pharmaceutical company that supplied the raw material. These products may correspond to the Rx or OTC category.
In any case, they have to be clearly identified from the production to the bedside.
2-2. Medical Devices
Medical device means any instrument, apparatus, implement, machine, appliance, implant, in vitro reagent or calibrator, software, material or other similar or related article intended by the manufacturer to be used, alone or in combination for human beings for one or more of the specific purposes of:
- Diagnosis, prevention, monitoring, treatment or alleviation of disease
- Diagnosis, monitoring, treatment, alleviation of or compensation for an injury
- Investigation, replacement, modification or support of the anatomy or of a physiological process
- Supporting or sustaining life
- Control of conception
- Disinfection of medical devices
Providing information for medical purposes by means of in examination of specimens derived from the human body and which does not achieve its primary intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
Healthcare Product Marking -3
When adopting the GS1 standards for Healthcare product marking consideration should be given to the following:
- AIDC Marking Levels
- Product Configuration
- Package Hierarchy
- Distribution Channel
The following sections will more fully describe these areas of consideration to be used by the organisations responsible for selection of the AIDC marking.
3-1. AIDC Marking Levels
The AIDC marking of healthcare products uses a graduated system of marking; Minimum, Enhanced and Highest. The identification solution for each of these levels may differ between the category of “pharmaceuticals” (which includes biologics, vaccines, controlled substances, clinical trial pharmaceuticals and therapeutic nutritional products) versus the category of “medical devices” (which includes all classes of medical devices) and may also differ by configuration or packaging level (trade items direct marked, primary packaging, secondary packaging, case/shipper, pallet, logistics unit).
Consideration should first be given to the data to be included in the data carrier. Decisions will take into account different levels of AIDC Marking. The three levels of AIDC Marking are designated Minimum, Enhanced and Highest based on information need.
3-1-1. Minimum Level of AIDC Marking
A level within a graduated system of AIDC trade item marking that provides a GTIN with no attribute information; it is used with products that do not require a high level of traceability control. Marking at this level includes the GTIN at a minimum.
3-1-2. Enhanced Level of AIDC Marking
A level within a graduated system of AIDC trade item marking, it provides GTIN plus attribute information. This includes products that require a higher level of traceability control. Marking at this level includes the GTIN, Batch or Lot Control Numbers, and the Expiration Date (when applicable).
3-1-3. Highest Level of AIDC Marking
A level within a graduated system of AIDC trade item marking, it provides GTIN, serialisation, and potentially other attribute information. This includes products that require the highest level of traceability control. Marking at this level includes a GTIN, Serial Number and Expiration Date (when applicable).
3-2. Product Configurations
Consideration should be given to where on the product the data will be carried. Decisions will take into account different levels of product configuration.
3-2-1. Direct Part Marking (DPM)
Direct part marking (DPM) refers to a process of marking a symbol directly onto an item without packaging using an intrusive or non-intrusive method instead of applying a label or using another indirect marking process.

Figure 1- DPM Example
3-2-2. Primary Package
The primary package is the first level of packaging for the product marked with a data carrier either on the packaging or on a label affixed to the packaging.
- Primary package configurations may consist of:
- sterile packaging
- non-sterile packaging
- single item
- group of items
- combination of items for a single therapy (kit)

Figure 2- Primary Package example
Figure 3- Kit example
3-2-3. Secondary Package
Secondary packaging configurations consist of packages containing one or more single items in their Primary Packaging

Figure 4- Secondary Package examples
3-2-4. Case / Shipper and Pallet
These are packaging configurations that may be used as either trade items or logistic units. Case /shippers may contain one or more items in their Primary Packaging and/or Secondary Packaging. Pallets may contain one or more case /shippers.
Note: Only fixed configuration Pallets and Shippers may be identified with a GTIN.
Figure 5- Case / Shipper Example

Figure 6-Pallet Example
3-3. Package Hierarchy
Consideration should be given to the hierarchy of packaging. Each level of the package hierarchy presents different printing challenges in labelling (e.g. space, substrate, production line speed, etc.).
Each level also presents differing data information requirements: GS1 Identification Key or GS1 Identification Key plus additional data.
The GS1 System provides a variety of Data Carriers that will meet the combination of printing and data needs. This section will offer illustrations and examples.
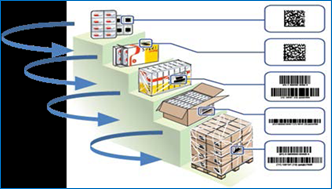
Figure 7- Package Hierarchy Illustration
The table below provides examples of the different symbologies, coding options and information attributes for each packaging level and the associated label size.
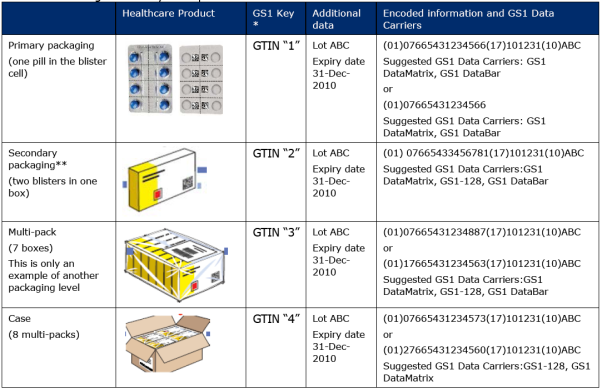
Table1- Package Hierarchy Examples
3-4. Distribution Channel
Consideration should be given to the channel (such as Retail Point-of-Sale) through which the products are intended to be distributed to aid in the selection of the data and data carrier. A product may belong in more than one channel depending on the target market(s).
Note: Specific sectors have requirements or restrictions with regards to the symbologies that may be deployed (e.g. Retail Point-of-Sale).
-
GS1 Identification Keys and Additional Data
When marking Healthcare products, GS1 Identification Keys are used to identify trade items and logistic units. Additional data may be associated with the GS1 Identification Keys through the use of GS1 Application Identifiers (AI’s). It is not a requirement to use multiple forms of product identification (GTIN, Global Returnable Asset Identifier (GRAI), etc.) on the same package.
4-1. Global Trade Item Number (GTIN)
The GS1 Key for unique product identification is the Global Trade Item Number (GTIN) which is assigned AI (01). GTINs are used to identify “trade items” (i.e., products and services that may be priced, ordered or invoiced at any point in the supply chain). They are assigned by the responsible entity who is normally responsible for the allocation of the GTIN.
A company receives a GS1 Company Prefix by joining a GS1 Member Organisation. This gives the company the ability to create GTINs and access to the GS1 standards.
4-2. Serial Shipping Container Code (SSCC)
The GS1 Key for uniquely identifying a logistic unit is the SSCC, which is assigned AI (00). The SSCC can provide a link between the physical logistic unit and information pertaining to the logistic unit that is communicated between trading partners using Electronic Data Interchange (EDI).
4-3. Global Returnable Asset Identifier (GRAI)
The GS1 Key for uniquely identifying returnable assets is the GRAI which is assigned AI (8003). GRAIs identify reusable packages or transport equipment of a certain value, such as a tote, a gas cylinder, or cold chain container. The GRAI enables tracking as well as recording of relevant data. A GRAI can be used to identify identical returnable assets (as an asset type) and when paired with an optional serial number can be used to uniquely identify individual assets within that asset type.
4-4. Global Individual Asset Identifier (GIAI)
The GS1 Key for uniquely identifying individual assets is the GIAI, which is assigned AI (8004). The GIAI enables tracking as well as recording of relevant data.
4-5. Batch/Lot – AI (10)
If you require additional data to identify Batch or Lot Number, the GS1 Application Identifier (10) is used and typically assigned at the point of manufacture. This additional data is alphanumeric with a variable length of up to 20 characters.
4-6. Expiration Date – AI (17)
If you require additional data to identify an Expiration Date the GS1 Application Identifier (17) (often referred to as expiry date, use by date or maximum durability date) is used. This indicates the limit of consumption or use of a product (e.g., for pharmaceutical products it can indicate the possibility of an indirect health risk resulting from the ineffectiveness of the product after the date). It is expressed as year, month and day (YYMMDD).
4-7. Expiration Date and Time – AI (7003)
When the exact expiration date and time is critical to patient safety, the Application Identifier (7003) is used. Date is expressed as year, month and day (YYMMDD) and time is expressed in hours and minutes (HHMM). Examples might be customised pharmaceuticals in a hospital or public pharmacy with an expiration time within a single day.
4-8. Serial Number – AI (21)
If Healthcare products are to be individually tracked and traced using a Serial Number, Application Identifier (21) can be used. This additional data is alphanumeric with a variable length of up to 20 characters.
4-9. Active Potency – AI (7004)
If use of a Healthcare product requires recording of active potency, then AI (7004) can be used to indicate that the additional data field contains an active potency. The active potency of certain biologics, such as haemophilia products, varies by batch from the nominal potency of the trade item and is measured in International Units (IUs).
5-Implementation Procedures
Management of product identification is the responsibility of the person or persons within the healthcare supply chain who are responsible for the AIDC marking of healthcare products, assets and/ or logistics units, such as:
- Manufacturer
- Wholesaler / Distributor / Re-packager
- GPOs
- Logistics providers
- Hospital / Pharmacy
5-1. How to Implement GTINs using as Barcodes
Before a GTIN implementation project is commissioned, executive support will be necessary. A statement of work should be developed to clearly describe the purpose, scope, goals, objectives, deliverables, and cost of implementation. An important consideration should be the analysis of the current master data, including the development of rules for configuration management and interchangeability. The GTIN will need to be mapped to the master data in a logical standardised fashion. Master data should be cleaned before GTIN assignment. Corporate standards and policy should be developed to regulate how the organisation will allocate GTINs moving forward and at what phase of product development lifecycle they get assigned. Systems for allocation of the GTIN and governance of master data creation are essential to ensure GTIN allocation is in accordance with the GS1 System.
5-1-1. Allocate GTINs according to the GS1 Healthcare GTIN Allocation Rules
To manage your GTINs accurately you should undertake the following:
- Allocate a unique Global Trade Item Number (GTIN) for each product and for each packaging level.
- Register the GTINs in a database linking the number to the product name, description and its hierarchy.
- The GTIN should never be duplicated or re-used even if the product becomes obsolete.
- Build in a quality process for GTIN allocation for new products to ensure that duplication does not occur.
- Build an automatic check digit calculator into your system.
5-1-1-1. Allocation of GTINs
The “Each” (a “base” unit) is a fundamental concept for GDSN (catalogue) and corresponds to the smallest saleable unit (historically more often than not… GTIN 12 or 13)
- In the retail market, the smallest unit will be scanned at the “Retail PoS” (Point of Sale)
- In the Healthcare industry, there is a “smaller” or “lower” unit, which will be scanned at the “Healthcare PoC” (Point of Care)
- Commonly referred to as the “Level Below the Each”
A wide range of global Healthcare experts contributed to the discussion and direction
- Single unit – Single item of medicine or medical device without any package, for example the single tablet or capsule in a blister or bottle, a syringe, etc.
- Single unit package / blister – Pack or package that contains one discrete pharmaceutical dosage form. i.e. a tablet, a certain volume of a liquid or that is the immediate package for a medical device (e.g. a syringe, etc.)
- Multiple unit blister/package – Immediate package for a medicine with more than one single unit. Package which fully encloses the pill / caplet / capsule. Each dosage form may be individually packaged. The individually blistered dosage forms are attached to each other in one strip. GTIN usually with other data attributes (e.g. batch/lot, expiry, serial number, etc.) which are generally voluntary
- Unit of Use – Refers to an individual unit package that is used to make up the patient specific prescription that is prescribed for administering to a patient. Unit Dose is the prescribed dose for a patient (while single unit relates to the product) and was Out of Scope for the LBTE work.


5-2. Small instrument marking
Marking process and technique must be matched to the individual items being marked (materials, sizes, shapes, etc.)
Ratified GS1 Standards for direct part marking of small surgical instruments were needed to ensure the traceability of instruments in a reprocessing (repair, sterialization, etc.) cycle.
Specific marking needs to manage critical internal logistics processes (use, cleaning, (dis)assembly, sterilisation, etc.)
- must fit on small space
- must be able to carry sufficient information (item identifier & serial number) to enable traceability
- must remain readable throughout the intended life span of the item
- must be practical (easily retrievable, etc.)
- must be biocompatible
- must be standards-based
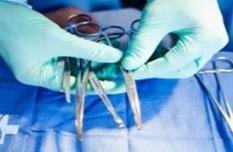
5-2-1.Data carrier: GS1 DataMatrix
Depending on the available space on the product, existing regulations and customer’s requirements, you will choose either a linear barcode or 2D symbol.
For small instrument marking, it’s better to use 2D symbol data matrix.
- Target useable mark area of 2.5mm x 2.5mm
- One bar code on a single instrument
- Though not limited to, laser etching is recommended
- Mixed marking technologies within the same scanning environment should be avoided (ensures highest reading performance)
The information that will be carried in the barcode and the type of symbology is determined by a number of factors. The following questions can be used in determining the information that should be carried in the barcode.

6-Conclusion
Automatic Identification and Data Capture
(AIDC) technologies will play a vital role in Improving supply chain efficiency through increased visibility.
Integrating data and using the same language across the supply chain will reduce transaction and processing costs. It will also reduce manual data capture, double checking, and relabeling, while increasing the accuracy of these processes.
Effectively sharing master data of healthcare products between supply chain partners sets the foundation for an interoperable, electronic supply chain system and electronic procurement systems.
Using AIDC Application Standards in healthcare helps to improve patient safety and increase efficiency and save costs.
- Improving patient safety
- Reduce medical errors
- Enable effective product recalls
- Fight counterfeiting
- Enable adverse event reporting
- Increase time for patient care
- Increase efficiency & save costs
- Improve order and invoice process
- Optimise receiving
- Reduce inventory and improve shelf management
- Increase productivity
- Improve service levels/fill rate
- Improve benchmarking and management of supply cost
- Efficiently document treatment in patients’ Electronic Health Recor



 صاحب امتياز
صاحب امتياز