ماهنامه شماره14(مهر ماه 1395)
By: Maryam Pourahmadi
1. Introduction
Business processes in a large variety of industries are becoming increasingly digitized. In the new era of the paperless office, it is not uncommon for all kinds of data to be transferred electronically rather than on paper. The healthcare industry is no exception, and electronic attachments for insurance claims are just one type of EDI in healthcare. But what is EDI? Electronic data interchange is more than just email; it is a structured way to transmit data between computer systems, governed by standards that are extremely important for medical claims.
Organizing and sending data between parties in the medical and dental industries has always been a complicated process, particularly in the management of both patient medical records and health insurance reimbursement details. However, by increasing in efficiency, EDI implementation has proven to both save time and save money. An important element in EDI is that of standards. Each EDI document has a standardized format, which ensures that data can be quickly sent and interpreted on both sides. EDI formatting specifications are like blueprints for data, EDI guides that serve to make transitions between different data trading partners as smooth as possible.
The reason that EDI has become especially important with respect to insurance claim documentation is the proven increase in efficiency seen with the use of electronic attachments.

2. GS1 EDI
GS1 EDI is a set of global electronic messaging standards for business documents used in Electronic Data Interchange (EDI). GS1 EDI is part of the overall GS1 system, fully integrated with other GS1 standards, increasing the speed and accuracy of the supply chain.
GS1 developed the following sets of complementary EDI standards:
- GS1 EANCOM – a subset of UN/EDIFACT, which comprises a set of internationally agreed UN standards, directories and guidelines for EDI. EANCOM is fully compliant to UN/EDIFACT.
- GS1 XML – a GS1 set of electronic messages developed using XML, a language designed for information exchange over internet. GS1 XML is based on UN/CEFACT Core Component Technical Specification (CCTS) and UN/CEFACT Modeling Methodology (UMM).
- GS1 UN/XML – GS1 has also developed its own profiles of four UN/CEFACT XML standards (Cross Industry Order, Order Response, Invoice and Despatch Advice), which are fully compliant with UN/XML.
3. GS1 EDI in healthcare
GS1 EDI standards are used throughout the Global Healthcare supply chain from supplier to logistics end-user.
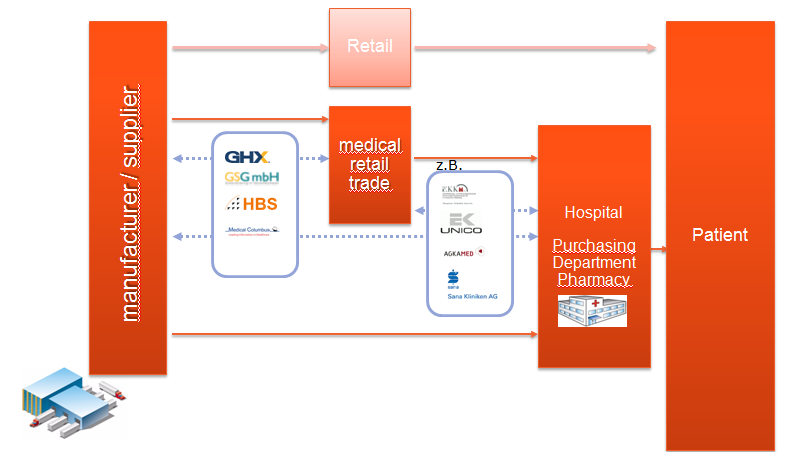
Figure 1: EDI Implementation in HC
In the following figure, the information sharing has been shown in two flows; Information flow and Physical flow. The information sharing can be conducted through three ways according to the data types.
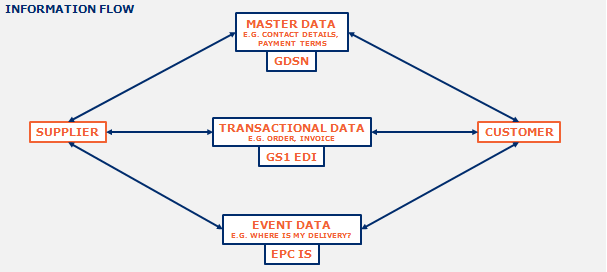
Figure 2: Information sharing
GS1 has published an implementation kit for EDI. This kit explained the key steps to how healthcare industry and its stakeholders implement the EDI. In figure 3, we can see the healthcare business process model which have been published in this guideline.
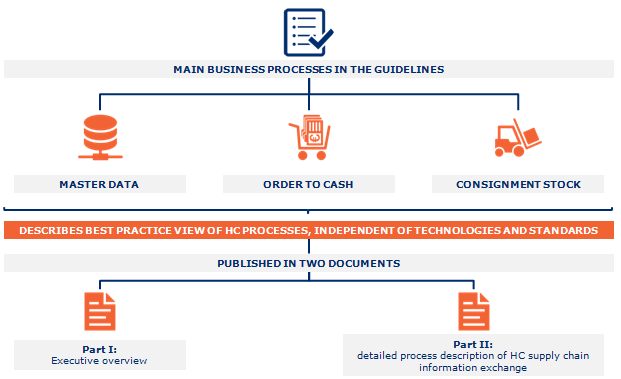
Figure 3: business process model
This is master data that needs to be agreed and exchanged before the EDI (transactional) exchange starts. Example of contract details could include:
- How often deliveries are to be made.
- When the supplier expects to be paid (usually when delivery has been made).
- Terms of delivery – when responsibility is transferred from the supplier to the buyer.
- Freight charges
The process of order to cash by using GS1 EDI and business transactions and the processes between the suppliers and buyers can be seen in the figure 4.
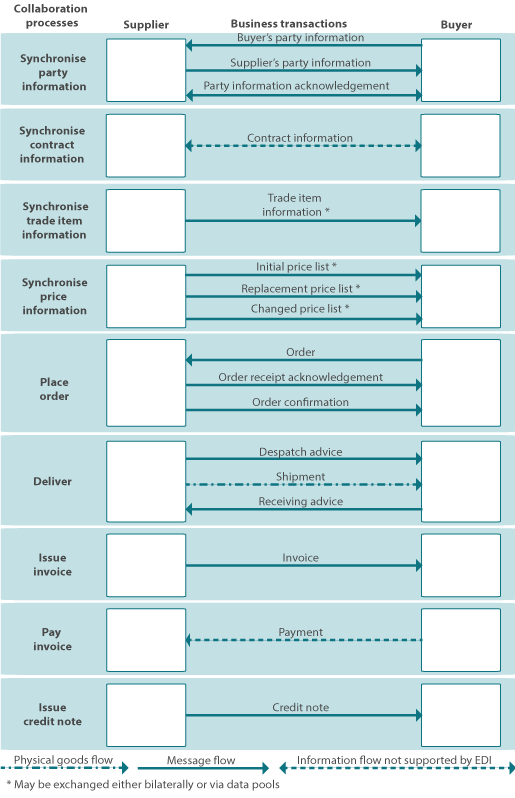
Figure 4: process of order to cash
3-1. The healthcare supply chain
The business processes in supply chain can be described in five steps, demonstrated in figure 5.
Below we discuss about each steps.
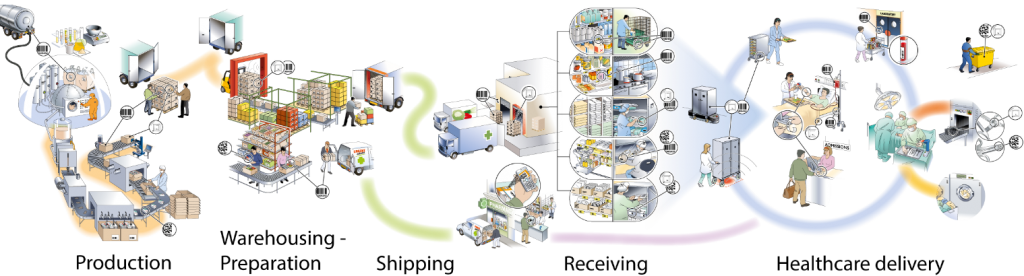
Figure 5- healthcare supply chain
3-1-1. Production
The raw material is delivered to the production site and products are manufactured.
The information from the despatch advice is used in combination with the identities of the logistic units (SSCCs) to confirm that the right quantities have been delivered. The GTIN and batch/lot number of the raw material are read and registered.
The registered GTINs and batch numbers of the raw material are used in the manufacturing process to enable traceability from the product back to the raw material site.
Each packaging level of the manufactured products is assigned a GTIN. The products are marked with batch/lot numbers and dates, and the information is registered in order to achieve traceability in the next stage of the supply chain. The logistic units are marked with identities (SSCCs), and this information is also registered in order to achieve traceability at the logistic unit level.
3-1-2. Warehouse Preparation
The products are received and stored at the warehouse.
During the storage period, physical inventories are performed. Upon receipt of a customer’s order, the ordered products are picked and logistic units are created and made ready for shipping.
Product arrivals can be managed using the identities of the logistic units (SSCCs). During storage, physical inventory can be carried out using the GTINs and batch /lot numbers of the products and the identities of the logistic units (SSCCs). Inventory management can be optimised using batch/lot numbers.
Orders may be sent electronically. Each logistic unit created at order picking is assigned an identity (SSCC). Traceability can be achieved by connecting the SSCCs with the identity of the recipient of the goods (GLN), the identities of the products (GTINs) and the batch/lot numbers.
3-1-3. Shipping
The logistic units are loaded onto the transport vehicle.
The vehicle leaves the warehouse. When the logistic units are loaded onto the transport vehicle the identities (SSCCs) are read and registered. Before the transport vehicle leaves the warehouse, a despatch advice is created and sent to the goods recipient. This enables more efficient and effective delivering, goods receipt and invoicing processes.
3-1-4. Receiving
The despatch advice is received prior to the goods.
The goods are received and reconciled and the inventory records are updated when the transport vehicle arrives. Planning for the receipt of goods can be efficiently managed based on the despatch advice. Upon receipt of the goods the identity of each received logistic unit (SSCC) is read which enables an automatic connection to the despatch advice. In this way the subsequent control and payment of the invoice can be automated through matching to the relevant order.
3-1-5. Healthcare delivery
At a hospital there are many internal processes, all aiming to provide quality care to patients.
The internal processes are more efficient and secure by using the GTINs of the products and the identities of the internal functional units (GLNs). The internal processes use the same data, e.g., serial number or batch number, as was received when the goods were delivered, which creates traceability backward through the supply chain.
Patients, and the care provided to them, e.g., surgical operations, blood transfusions, X-ray treatments and medications, are identified using the Global Service Relation Number (GSRN), which is read and registered in a database at each stage and movement of the patient, during her hospital stay. Therefore, the GSRN contributes to the safety and traceability of the patient.
4. GS1 EDI and global standards implementation barriers
There are a few barriers when it comes to companies adopting GS1 EDI standards. Below we discuss these barriers.
4-1. Healthcare companies not aware of the benefits.
Today, many pharmaceutical and medical device manufacturers, as well as wholesalers and distributors leverage global standards for data exchange. More and more hospitals and retail pharmacies are also starting to realise the benefits as GS1 standards become a foundation for collaboration. This collaboration in turn drives capability to implement new processes, which benefit all parties in the trading relationship including ultimately, the patient. Examples include:
• More effective inventory management resulting in awareness of product availability across trading relationships
• Automation of transactional data sharing, removing the need for manual data entry and resulting error correction, reducing costs.
For more information about the benefits, review the EDI in Healthcare Value Proposition in the previous pages.
4-2. Why move to EDI? After all, email is easier.
Indeed, both are computer-to-computer exchanges and both use an electronic mailbox. However, email messaging format is not based on a standard. Email requires a human interface and is not acceptable to applications; whereas, EDI requires standard message formats between trading partners. The ability to send business documents between machines simplifies and expedites the business process. Many healthcare companies choose EDI as a fast, inexpensive, and safe method of sending purchase orders, requests for quotations, quotations, invoices, payments, and other frequently used business documents.
Standards are a necessary and important part of EDI communication. Every business has application files that are used to manipulate their data in ways that are familiar to the business. The problem is that most businesses, even using the same types of data, do not use the same application programs or hardware and software platforms. If businesses need to be able to communicate data to one another, they must have common ground to allow the exchange of information. Standards provide the solutions to this problem. All businesses that conform to specific standards can share data in the formats delineated by those standards.
This is where GS1 EDI standards come in. GS1 standards are the best choice for linking healthcare organisations in the private and public sectors.
Users receive support from their local GS1 MO in their own language, according to their local business needs.
4-3. Not sure which standards are the best.
• GS1 has 40 years of experience in standards for supply and demand chains worldwide
• GS1 EDI standards provide solutions for multiple healthcare stakeholders
• GS1 EDI development is based on an organised process
• The development and modifications of GS1 standards follow a rigorous, well-documented change management procedure: the Global Standards Management Process (GSMP)
• Each GSMP step includes broad user involvement and is validated by users regarding the relevance of the change and commitment to implement
• The final solution is checked for compatibility with other GS1standards and approved by the users
Existing business processes built around sluggish paper handling may not be suited for EDI and would require changes to accommodate automated processing of business documents.
The full implementation of GS1 EDI messages for the order to cash process can help solve this problem. Despatch and receiving advices, combined with the scanning of healthcare products during despatch and receipt, help to generate an invoice based on products that have actually been sent and accepted.
4-4. Cost in time and money
The preliminary expenses and time that arise from the implementation, customisation and training can be costly and therefore may discourage some businesses.
The key is to determine what method of integration is right for the company which will determine the cost of implementation.
For a small healthcare company or supplier that only receives few orders per year from a hospital of pharmacy, fully integrated EDI may not make economic sense.
In this case, businesses may use an inexpensive solution such as web EDI provided by EDI solution providers.

4-4-1.Cost and savings
Cost and savings calculators are important tools to help companies implementing EDI to determine the investment and return from the implementation. Some GS1 Member Organisations have developed cost and savings calculators that demonstrate that using GS1 EDI in Healthcare brings competitive advantages.
As an example, GS1 Australia in collaboration with its members, including retailers, buying organisations and suppliers have developed and tested a Cost and Savings calculator as detailed below.
- Measure the value
Calculate the potential economic benefits from implementing GS1 standards throughout your supply chain with the GS1 Savings Calculator.
- Four Simple Steps to Savings
The GS1 Australia Savings Calculator is a tool designed to assist all trading partners in the supply chain from suppliers and retailers to third parties, who want to identify the potential savings their organisation could extract by implementing GS1 standards.
Figure 6: four steps to savings
- The Challenge
Manual, paper based processing of business transactions exchanged between trading partners (such as product master data, purchase orders, delivery advices and invoices) leaves much room for human error. It can be very costly and time consuming.
Adopting a standards based approach to paperless trading across all these processes will create business value for your company, your business partners and ultimately the end consumer or end user.
The impact of automated order-to-cash processes in the supply chain is particularly tangible: more fluid stock movements, greater flexibility and accuracy in stock management, improved traceability and better product availability.
To help companies measure the potential savings, GS1 Australia has created the Savings Calculator. The Savings Calculator is a decision-making tool which allows each company to assess the benefits of using GS1 standards based on their own supply chain processes and variables.
GS1 Australia has leveraged the work completed by GS1 UK and GS1 France. They have also collaborated with Deakin University and Cranfield University UK to ensure the Savings Calculator delivers accurate results.
A number of GS1 Australia members, including retailers, buying organisations and suppliers have also assisted in the development and testing of the Savings Calculator.
- The Benefits/Proven Results
Order-to-cash automation with GS1 gives companies a competitive advantage:
• Cutting operational costs to the business
• Eliminating errors in processes
• Shortening delivery time
• Reducing out of stocks
• Significantly lowering distribution costs
• Improving customer satisfaction
5. EDI implementation drivers in healthcare
Drivers for the implementation of EDI in healthcare may vary between markets as a result of internal and external factors. However, they generally fall into the following categories:
- Helping to ensure quality of care
- Meeting regulatory or trading partner requirements
- Facilitating product traceability
- Increasing supply chain efficiency and accuracy and reducing costs
- Enabling new business processes
- Helping to ensure quality of care
The use of EDI ensures more accurate communication about products ordered, shipped and received throughout the healthcare supply chain. This is particularly important in a hospital or pharmacy environment when supply chain miscommunication or errors could mean the product needed for patient treatment is not available at a critical time, potentially risking quality of patient care and outcomes. For example, a required, yet unavailable product may mean a delayed surgery.
This leads to patient stress and confusion, increased risk of contracting an unrelated infection due to a longer stay in the hospital, and financial impact for the patient and their family.
Additionally, the hospital and healthcare system is now faced with an unused operating theatre, clinical staff members who must be reassigned to alternate tasks, and an expanding waiting list since the original surgery must be rescheduled. All of these disruptions and changes to the flow of services throughout the hospital may have a negative impact on the safety and health outcomes of other patients since their surgeries may also be delayed.
- Meeting regulatory or trading partners requirements
Many trading partners have realised the broad-scale benefits of EDI-driven transactions—either to reduce costs or combat fraud—and are, therefore, requesting that their trading partners become EDI enabled. In response, a significant number of companies are implementing EDI to meet their trading partner requests.
- Facilitating product traceability
EDI messages can include information identifying the specific product being discussed as well as information about the product’s batch / lot or even serial number. Where traceability of products is important, this detailed product data helps ensure accurate and complete electronic records about the specific products ordered and shipped to a particular trading partner.
In the case of a product recall or withdrawal, this information can then be used to efficiently track the product to its current location, accelerating its removal from shelves.
- Increasing efficiency & accuracy and reducing costs
The use of EDI automates manual processes, thus eliminating the need for paper, printing, physical storage of documents and postage for increased efficiencies and cost savings. Electronic documents can also be processed more quickly than those requiring manual intervention, ensuring that customer needs are met for higher customer satisfaction.
Inventory levels can be more effectively managed due to the reduction in lag time between the receiving and processing of order-to-cash documents. In addition, invoices and other financial documents can be processed in a timelier manner for increased cash flow.
- Enabling new business processes
Beyond making existing processes more efficient, EDI is a potent enabler for creating totally new business processes and supply chain solutions. Features like reliable communication, high-quality product data and near real-time processes are the foundation for solutions such as vendor managed inventory (VMI), automated reconciliation of invoices and traceability systems.
6. The benefits of GS1 EDI in healthcare
Ordering, delivering and paying for products is what most healthcare companies do.
With GS1 EDI we can improve speed, Accuracy, Efficiency and also save time and money.
Speed
• Instant exchange of business data
• Lower operating costs: Saves time and money
Accuracy
• No risk of data entry error
• Less errors means greater accuracy due to no data entry and less human error
Efficiency
• Seamless integration of complete supply chain processes
• Increased productivity; more efficient personnel and faster throughput
• Faster trading cycle with streamlined processes for improved trading relationships
Time and money savings
• Buyers, sellers and other participants save time and money when the business process, from contract to invoice, is carried out electronically and according to agreed upon rules
• Facilitate system integration and reduce development costs
• Enable and ease integration of new business partners
• Consistent business processes mean quality of care and patient safety are improved
7. Case studies of implementing EDI in Healthcare
7-1. Using GS1 standards to improve EDI accuracy and achieve the perfect order
In 2011, Becton, Dickinson and Company (BD), Mercy Health (Mercy) and its supply chain company Resource Optimization & Innovation (ROi) launched a collaborative initiative to fully automate their order-to-cash process to achieve the “perfect order,” implementing GS1 standards from manufacturing site to patient bedside. This integration of global data standards—in supply chain and clinical processes—by a healthcare manufacturer and provider is a first-time accomplishment in the U.S. healthcare industry. Moving forward, the trading partners have continued to perfect and extend their perfectorder success, resulting in highly accurate and efficient processes with a continual focus on improving patient care. This review will provide an update on how the two organisations implemented EDI to achieve supply chain efficiencies and how their use of GS1 standards continues to evolve
7-2. Optimising business operations with GS1 EDI business processes
In today’s healthcare market, competitive pressure, regulatory requirements and new ways of working are demanding that manufacturers and retailers collaborate more. In 2013, Coopidrogas started implementing GS1 EDI transactions in order to optimise its members’ business operations by lowering costs, increasing speed and improving accuracy and business efficiency. As part of its new process, Coopidrogas has adopted GS1 EDI-based documents such as purchase orders, shipping notices and receiving advices, and uses the Global Data Synchronisation Network™ (GDSN®) to update its master database.
7-2-1. Background
Cooperativa Nacional De Droguistas Detallistas (Coopidrogas) is a non-profit association dedicated to the promotion of business improvements for independent pharmacies. It was created 46 years ago by a small group of independent pharmacy owners who, based on their small size and low-volume purchases, faced difficult negotiations with pharmaceutical suppliers, thus impacting their profit margins. Today, Coopidrogas has approximately 3,500 independent pharmacy owner members with 6,500 drugstores, representing approximately 41 percent market share in Colombia.
7-2.2. The need for complete, accurate data
Coopidrogas has more than 20,000 products, 367 suppliers and six distribution centres that serve 614 locations in major cities, townships and remote villages.
To maintain a lean and efficient supply chain, Coopidrogas faced three main challenges:
- Keep product information up-to-date in order to facilitate the flow of information between the independent pharmacies.
- Ensure the quality of data in order to support the implementation of technology and GS1 EPC-enabled RFID (EPC/RFID).
- Decrease the time dedicated to product coding by using EDI transactions for lower costs and faster time-to-market intervals.
7-2-3. Improving information flow across the value chain
The project developed by Coopidrogas focused on the implementation of GS1 standards and business-to-business (B2B) commerce based on GS1 EDI standards, to include product data synchronisation as well as electronic transactions like purchase orders, shipping notices and receiving advices. Designed to reduce time to market and guarantee data quality, the new solution aims to improve the information flow across the Coopidrogas value chain.
The solution was based on the following goals:
- Adopt GS1 standards using the GS1 Global Trade Item Number (GTIN), Global Location Number (GLN) and GS1 EDI technical specifications.
- Align commercial and logistics processes based on information received from B2B commerce with suppliers.
- Develop partnerships with companies that offer B2B-commerce services for an efficient way to engage suppliers.
- Connect to LOGYCA / SYNC, the GDSN-certified data pool in Colombia.
- Collaborate with the pharmaceutical industry through GS1 Colombia with the aim of defining basic criteria to provide accurate information and training to different stakeholders.
7-2-4. Accurate and complete data for all stakeholders
Stakeholders involved in the solution included pharmaceutical suppliers, Coopidrogas members of independent pharmacy owners and GS1 Colombia.
- In the pharmaceutical industry, Coopidrogas has 367 suppliers (laboratories). Participation and commitment from these suppliers is critical since they are the main producers of information and have the required knowledge about pharmaceutical products to ensure they are marketed correctly.
- The estimated 6,500 drugstores are benefiting, thanks to improvements based on the implementation of best practices in logistics that enabled them to receive products on time, reduce out-of-stock situations and improve profit margins.
- GS1 Colombia provided a neutral meeting place for the development of the collaborative initiatives based on global standards and the introduction of best practices in logistics. Coopidrogas is part of the GS1 Colombia Healthcare Group that brings together the main players in the country’s healthcare sector in order to make the healthcare value chain more efficient, flexible and competitive in areas such as data quality and the traceability of medicines. Pharmacies now collaborate with suppliers to monitor stock management indicators such as out-of-stock and service levels.
To date, achievements include:
- Technical information about the product is accurate and complete. More than 90 percent of Coopidrogas’ products are synchronised through the LOGYCA / SYNC data pool.
- There is perfect alignment of the master data between suppliers and retailers. During 2015, 70 percent of the products were synchronised using different GS1 EDI messages.

- Accuracy of purchase orders has increased from 92.9 to 96.4 percent in the first four months after the GS1 EDI implementation.
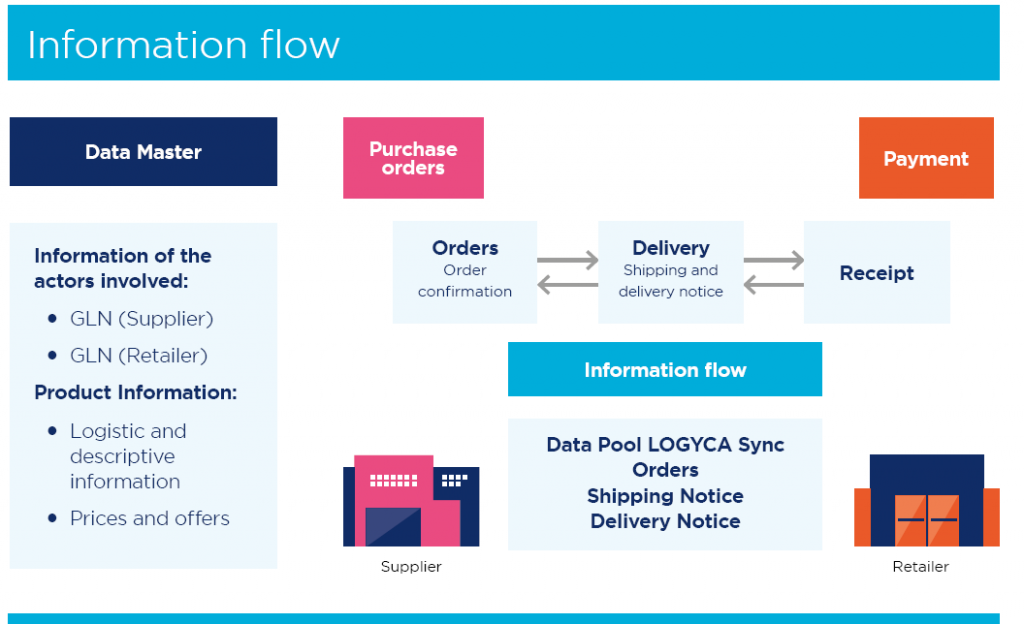
Figure 7. The information flow



 صاحب امتياز
صاحب امتياز